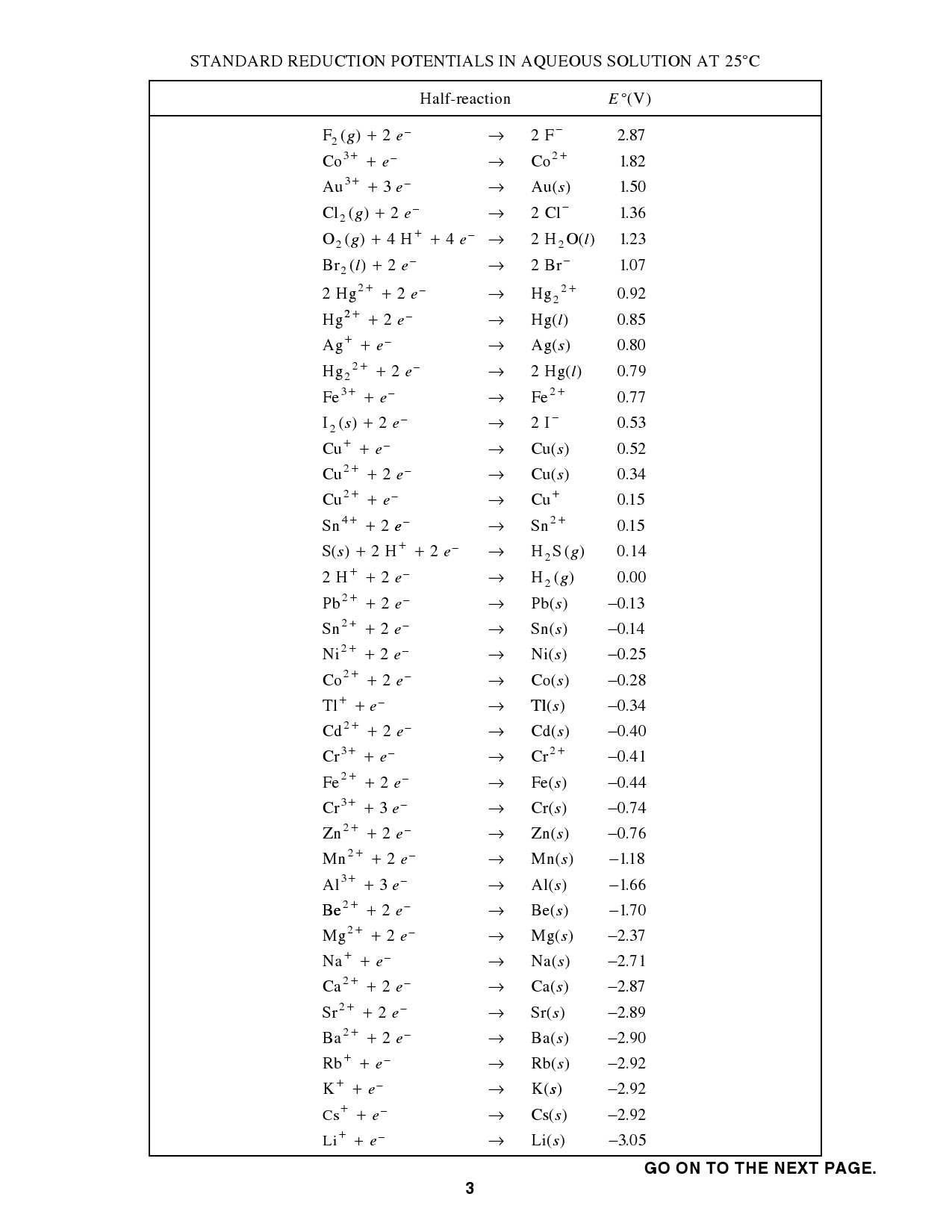
Standard Cell Potential Chart . we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. standard electrode potentials in aqueous solution at 25°c. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The following table provides eo for selected reduction. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard reduction potentials by value.
from www.albertgural.com
The following table provides eo for selected reduction. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. standard electrode potentials in aqueous solution at 25°c. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. Standard reduction potentials by value. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.
Equations Albert Gural
Standard Cell Potential Chart 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. standard electrode potentials in aqueous solution at 25°c. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Standard reduction potentials by value. The following table provides eo for selected reduction. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product.
From thechemistrynotes.com
Cell Potential & Standard Cell Potential Standard Cell Potential Chart we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. use standard reduction potentials to calculate a standard cell. Standard Cell Potential Chart.
From mungfali.com
Table Of Standard Electrode Potentials Standard Cell Potential Chart 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. standard. Standard Cell Potential Chart.
From www.myxxgirl.com
Half Cell Potential Table My XXX Hot Girl Standard Cell Potential Chart 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The following table provides eo for selected reduction. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict. Standard Cell Potential Chart.
From www.studypool.com
SOLUTION Calculating standard cell potentials Studypool Standard Cell Potential Chart we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. The following table provides eo for selected reduction. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to. Standard Cell Potential Chart.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Chart we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. The following table provides eo for selected reduction. Standard reduction potentials by value. the cell potential, \(e_{cell}\), is the. Standard Cell Potential Chart.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Chart 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The following. Standard Cell Potential Chart.
From www.vrogue.co
Electrochemistry Electrode Potential And Standard Ele vrogue.co Standard Cell Potential Chart Standard reduction potentials by value. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to. Standard Cell Potential Chart.
From www.chegg.com
Solved Relative HalfCell Potentials Assuming standard Standard Cell Potential Chart 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The following table provides eo for selected reduction. Standard reduction potentials by value. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 42 rows calculate the standard. Standard Cell Potential Chart.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Cell Potential Chart the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The following table provides eo for selected reduction. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. Standard reduction potentials by value. standard electrode potentials in aqueous solution. Standard Cell Potential Chart.
From mccord.cm.utexas.edu
Standard Potential Standard Cell Potential Chart standard electrode potentials in aqueous solution at 25°c. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. Standard reduction potentials by value. The following table provides eo for. Standard Cell Potential Chart.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Chart Standard reduction potentials by value. standard electrode potentials in aqueous solution at 25°c. The following table provides eo for selected reduction. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an. Standard Cell Potential Chart.
From mavink.com
Galvanic Potential Chart Standard Cell Potential Chart Standard reduction potentials by value. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict. Standard Cell Potential Chart.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Chart The following table provides eo for selected reduction. standard electrode potentials in aqueous solution at 25°c. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Standard reduction potentials by value. . Standard Cell Potential Chart.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Cell Potential Chart standard electrode potentials in aqueous solution at 25°c. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. The following table provides eo for selected reduction. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. Standard reduction potentials. Standard Cell Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Cell Potential Chart 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. standard. Standard Cell Potential Chart.
From turtaras.blogspot.com
Astonishing Photos Of Cell Potential Table Concept Turtaras Standard Cell Potential Chart The following table provides eo for selected reduction. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. standard electrode potentials in aqueous solution at 25°c. Standard reduction. Standard Cell Potential Chart.
From www.coursehero.com
Determine the standard cell potential for the reaction below of Standard Cell Potential Chart The following table provides eo for selected reduction. Standard reduction potentials by value. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 42 rows calculate the standard. Standard Cell Potential Chart.
From www.chegg.com
Solved C. Calculating Standard Cell Potentials, Eºcell Given Standard Cell Potential Chart standard electrode potentials in aqueous solution at 25°c. Standard reduction potentials by value. use standard reduction potentials to calculate a standard cell potential, e° cell, and predict whether a reaction is product. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 42 rows calculate the. Standard Cell Potential Chart.